Main Topics
Keywords: Clusters, Crystal Engineering, Alkali Metals, Alkaline Earth Metals, Group 11 Elements Cu, Ag, Au, Supramolecular Chemistry, Oxide Materials, Anti-microbial Biomaterials, Nanomaterials, Nanocontainers, Nanorattles, Nanocomposites, Molecular Magnetism, Bioinorganic Chemistry of Silver, Li-ion Battery Materials, Battery Assembly and Testing, Bacterial Resistance, Hydrogels, Luminecense Coordination Compounds, Biomarkers, Photoelectrocatalysis, CO2-Reduction, H2-Production.
Anthracene-based ligands and their coordination compounds

The optical properties of anthracene allow the development of molecular sensors, e.g. for indium or iron ions (A. Finelli, V. Chabert, N. Hérault, A. Crochet, C. Kim, K. M. Fromm, Inorg. Chem., 2019, 58, 20, 13796-13806. DOI: 10.1021/acs.inorgchem.9b01478) or to use them in sponge-like structures for the detection of molecular compounds, such as nitro-aromatics or pesticides (S. I. Vasylevskyi, D. M. Bassani, K. M. Fromm, Inorganic Chemistry 2019, 58, 9, 5646-5653. DOI: 10.1021/acs.inorgchem.8b03628).
Bacteria as electron donors
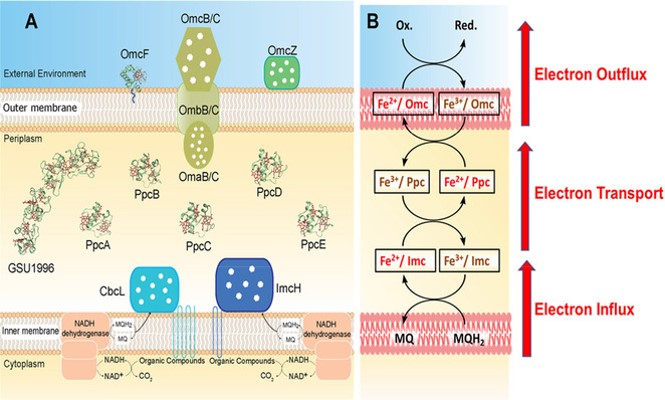
Since a few years, we have started to investigate the anaerobic Geobacter sulfurreducens, which are able to deliver electrons to their environment. To understand this mechanism and to quantify bacterial respiration, we study the electron transfer process through the bacterial membrane (V. Chabert, L. Babel, M. P. Füeg, M. Karamash, E. S. Madivoli, N. Herault, J. M. Dantas, C. A. Salgueiro, B. Giese, K. M. Fromm, Angew. Chem. Int. Ed., 2020, 59, pp 12331-12336.DOI: 10.1002/anie.201914873)
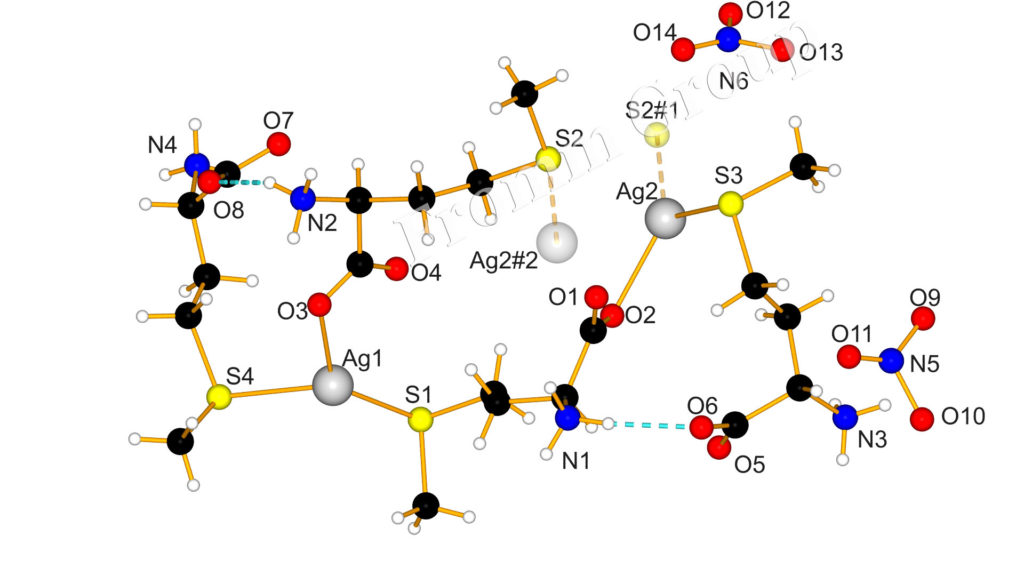
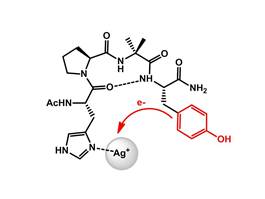
Bioinorganic Chemistry of Silver : Interaction of Silver with Peptides
Although the antimicrobial properties of silver have long been accepted, the molecular mechanism of action of silver with biomolecules is not established in detail. We are therefore interested in coordination and redox behaviour of silver-peptide compounds. A comprehensive review on silver, its ions and nanoparticles and what is known about how they interact with each other in cells and bacteria, is described in the following paper: Chem. Rev. 2013; DOI: 10.1021/cr300288v. While in another recent paper, we describe the interaction of silver with peptides and c-Cytochromes (Angew. Chem. Int. Ed. 2017; DOI: 10.1002/anie.201702621). S.-L. Abram, K. M. Fromm; Chem. Eur. J.2020, 26, 10948-10971. DOI: 10.1002/chem.202002143
Materials for Lithium Ion Batteries
We are dealing with low-temperature precursors for LiCoO2, and have succeeded in the production of this oxide at much lower temperatures (450°C) than the classical solid state high-temperature pathway (850-900°C). Apart from making such material at the nanoscale, we also investigate the assembly into electrodes and test electrochemical properties. We do this in collaboration with Dr. Nam Hee KWON, who works on nanoscale olivine structures and their use in electrodes for Li-ion batteries. (J. Power Sources 2017, 342, 231-240; DOI: 10.1016/j.jpowsour.2016.11.111).

Nanomaterials
Nano-structured surfaces play important roles in materials sciences (Chem. Eur. J. 2015; DOI:10.1002/chem.201405285). We use them in the field of biomaterials (see below). Silver nanoparticles can be made as a function of the amino acid sequences present during their generation (Angew. Chem. Int. Ed. 2009, 48(20), 3661-3664; ).

Ag-nanoparticles on polymer beads 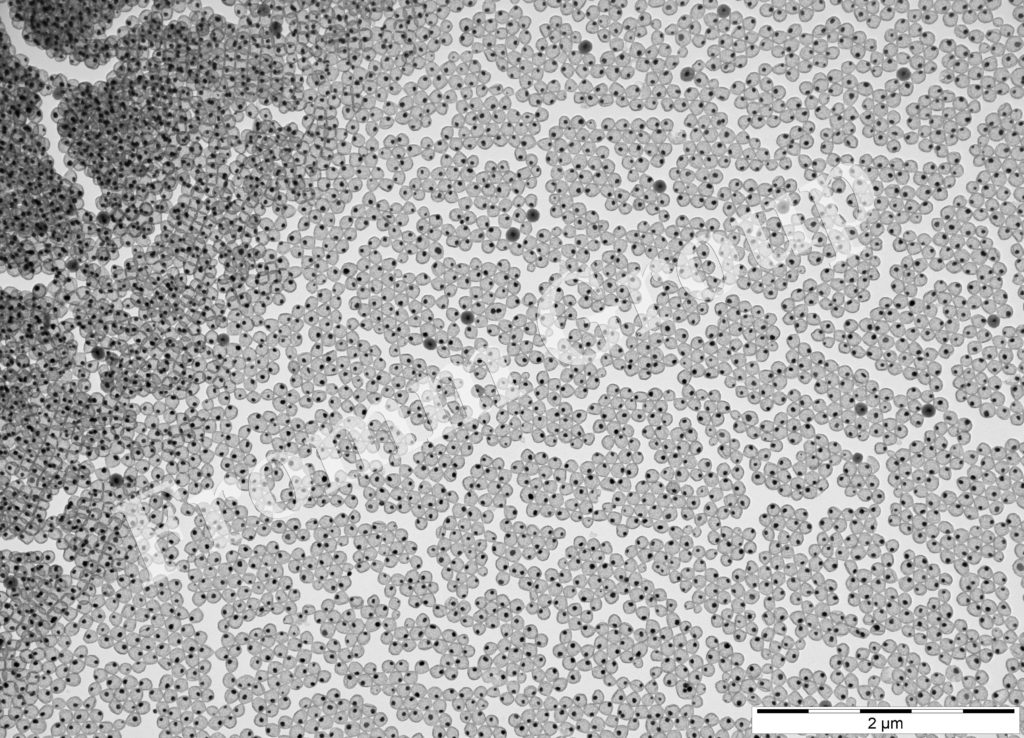
silver-filled silica nanorattles
Anti-microbial biomaterials and surfaces
The use of orthopedic implants has led to an increase in the absolute number of implant infections, triggering a search for more effective antibacterial coatings. Silver-containing silica nanorattles (Ag@SiO2) were evaluated for their antimicrobial potential and for their impact on cells of the immune system (Nanomedicine – Nanotechnol. Biol. and Med. 2017; DOI: 10.1016/j.nano.2016.08.002; Antimicrob. Agents Chemother. 2016; DOI: 10.1128/AAC.02934-15). The anti-microbial properties has also been studied with silver complexes: Comptes Rendus Chimie 2013; DOI:10.1016/j.crci.2013.04.010.
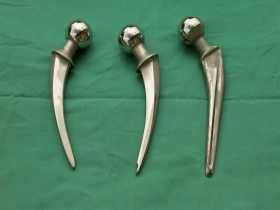
Artificial hip stems 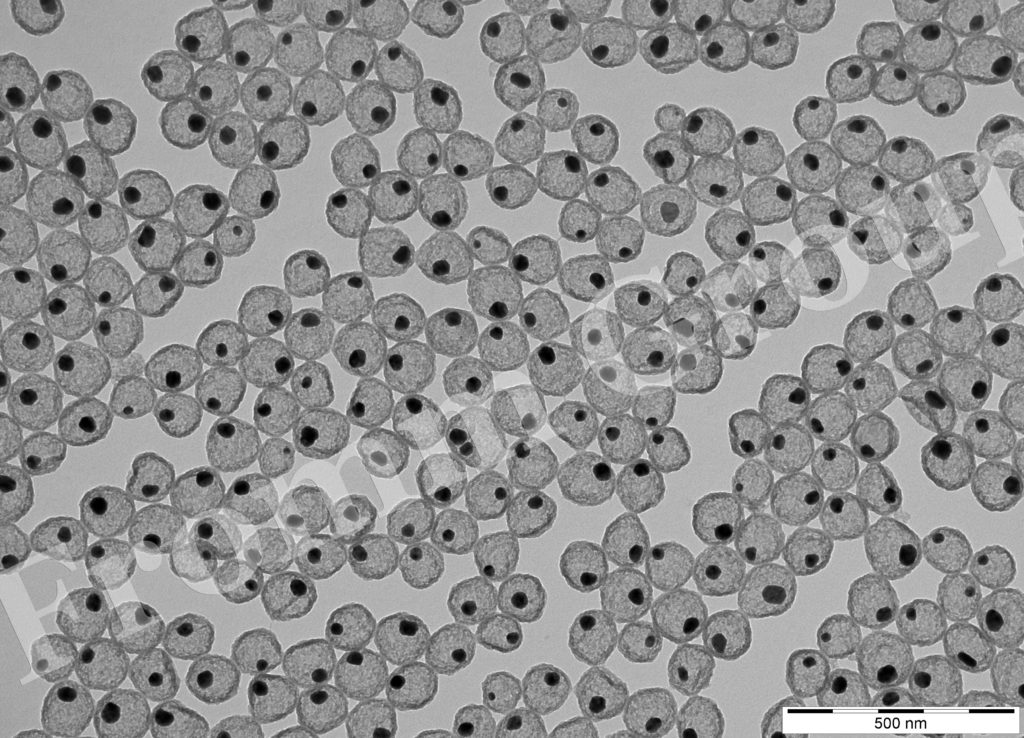
silver-filled silica nanorattles
Nanocomposites peptides and polymers with silver nanoparticles
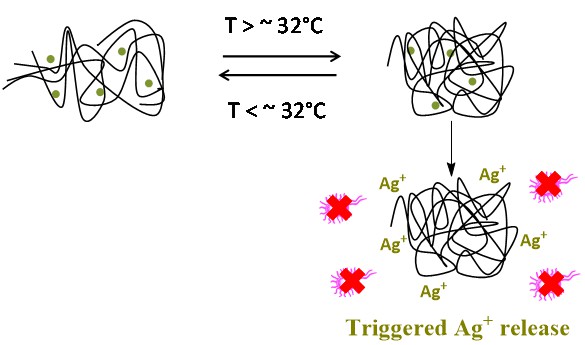
Our current work with peptides derivatives is focused on the analysis of beta-alanine dipeptide derivatives regarding their gel formation ability as well as silver ion coordination. This includes the dependence of temperature and formation of the Ag nanocomposite in derivatives containing a hydrazide. The synthesis on nanocomposite composed of polymers and silver nanoparticles is also a main subjet of our interest. We are currently working in the well-known thermoresponsive polymer (PNiPAAm) which could trigger the release of Ag+ and provide antimicrobial properties.

Coordination Polymers of Cu(I/II) and Ag(I)
Using ditopic ligands, we are able to generate mostly one-dimensional coordination networks with copper and silver ions. They are interesting from many points of view: polyelectrolytes, starting materials for mixed metal compounds due to the ligand’s different donor type atoms, as materials in coatings for disinfectant surfaces. (Coord. Chem. Rev. 2006, 250, 2127-2157; DOI:10.1016/j.ccr.2006.02.013; Cryst. Growth Des. 2016; DOI: 10.1021/acs.cgd.5b01084).
Mechano-responsive polymers
We are interested in the synthesis of new kind of stimuli-responsive polymers characterized by ferrocene derivatives as mechanophores. The aim is to selectively break in solution the sandwich complex under mechanical stress (ultrasounds) with the releasing of the central metal.
Electron Transfer through Peptides
Linked to the previous subject, we study – in collaboration with Bernd Giese – electron transfer processes through peptides, which are important in case of redox reactions occurring e. g. with silver (PhysChemChemPhys 2012, 14, 13785-13788; Angew. Chem. Int. Ed. 2017, 56, 5926 –5930; DOI: 10.1002/anie.201702621).
Oxide materials
Silver is one of the most powerful anti-microbial agents.We therefore investigate the deposition of our silver compounds on biologically relevant materials. Our alkali and alkaline earth metal compounds are tested for their potential use as precursors in oxide materials’ synthesis (Z. Anorg. Allg. Chem. 2005, 631, 1725–1740; ). Furthermore, we are interested in the antimicrobial properties of silver ions and their mechanism at the molecular level (see above).

Molecular Magnetism
We have come across some new chromium, cobalt and nickel cluster compounds which we are investigating for their magnetic properties. We are especially interested in compounds in which the metal ions are arranged in triangles, bridged by OR-groups. This is based on Kagomé lattices in which spin frustrated states can emerge. Watch out for our paper on pentagonal iron compounds in Eur. J. Inorg. Chem. 2012, 2012(16), 2725-2730;
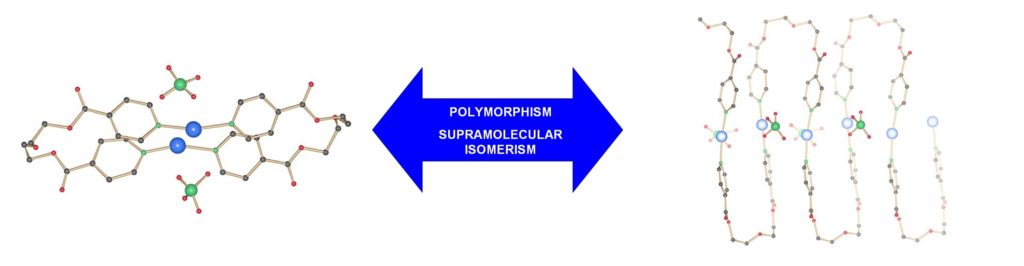
Polymorphism/Crystal Engineering
More generally, we are interested in polymorphism and pseudo-polymorphism, supramolecular isomerism and crystal engineering. In this field, many challenges remain that are related with the real structure prediction at the solid state, including weak inter-molecular forces (H-bonds, pi-stacking, metal-metal contacts, van der Waals interactions, etc.) and the process of crystallisation itself, which could be regarded as a very complicated process of self-assembly. (RSC Adv. 2013, DOI:10.1039/C3RA41559G; Chem. Commun. 2005, 36, 4548-4550, DOI:10.1039/B506389B).
Between Solid State and the Molecule: Alkaline Earth Metal Iodides
One of the more fundamental research themes in our group concerns the approach to “cut out” excerpts from solid state structures. In three-dimensional binary starting compounds with bond lengths of different length scale (as for instance in BaI2, where we have Ba-I distances varying from 3.4 to 4.2 Å), we can use chemical scissors in form of oxygen donor ligands to cut out three-, two- and one-dimensional compounds in which parts of the initial structure are maintained, whereas other bonds have been cut. Depending on the strength of the ligand and its concentration, we were able to derive for instance a structural genealogy tree for BaI2, cutting the structure down as far as to the molecular level (Angew. Chem.1997, 109, 24, 2876-2878, Angew. Chem. Int. Ed. Engl. 1997, 36(21), 2370-2372; Z. Anorg. Allg. Chem., 2001, 627, 1626-1630; ). K. M. Fromm, Coord. Chem. Reviews2020, 408, 213193. DOI: 10.1016/j.ccr.2020.213193
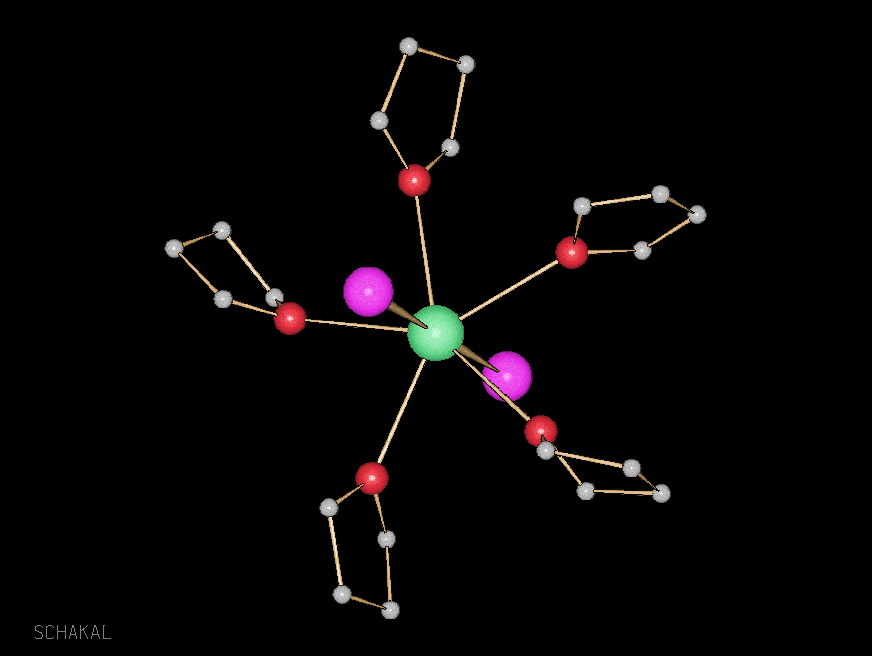
0-dimensional BaI2(thf)5 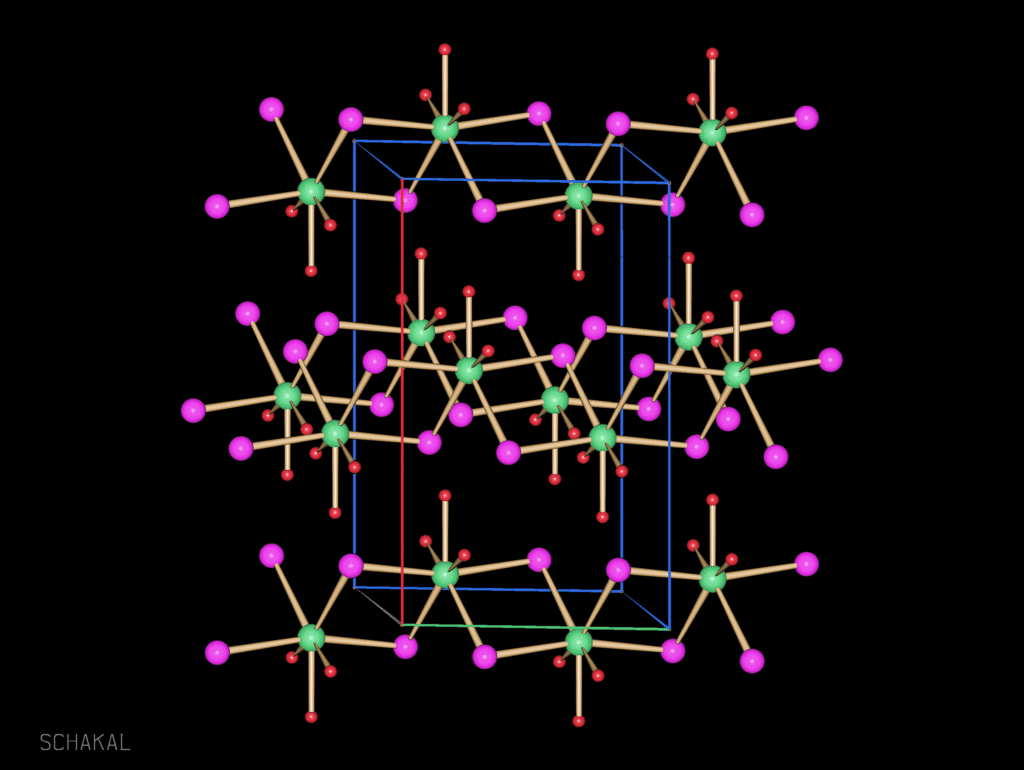
1-dimensional BaI2(thf)3 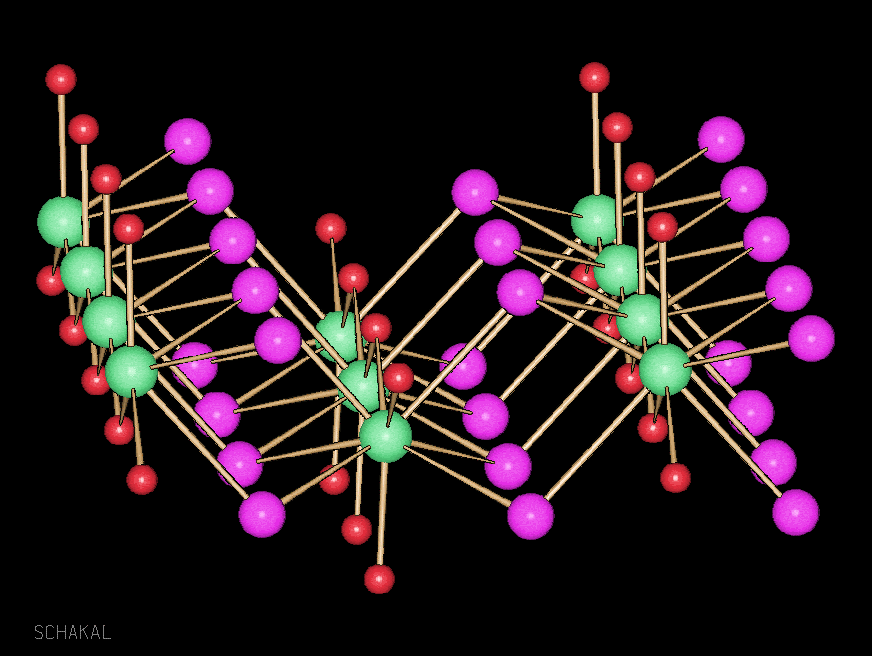
2-dimensional BaI2(OH2)(OC3H6)
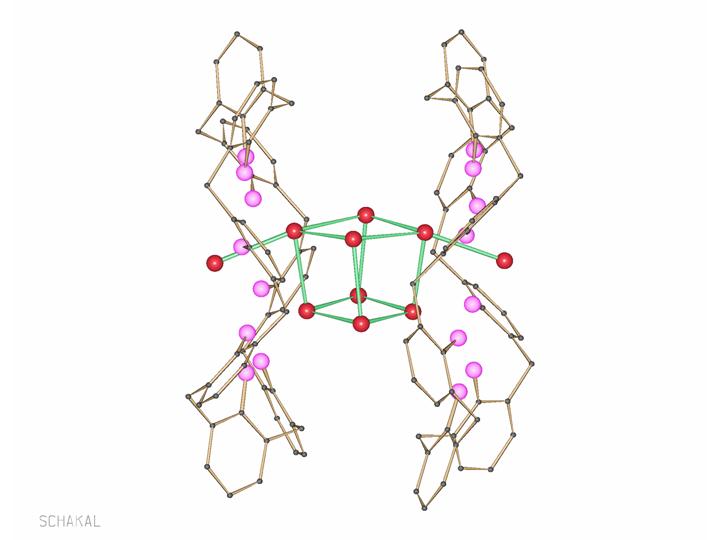
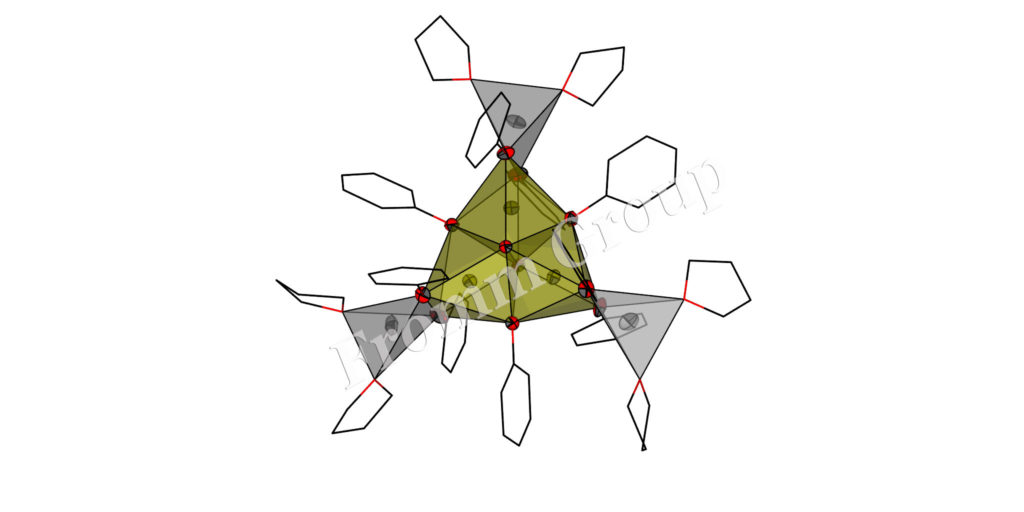
Clusters (Aggregates/Cage Compounds) with Alkali and Alkaline Earth Metals
Aggregates of alkali and alkaline earth metals were so far obtained mostly as secondary products in organic metallation reactions. We got interested in such compounds because they can be valuable precursors for oxide materials. A more fundamental aspect is the analogy to transition metal clusters. And in our group a developed synthesis allows isolation of a number of pure alkali and alkaline earth metal clusters, but also mixed metal aggregates. Some of them are useful in the sol-gel technique, others are volatile and yield halide and carbonate-free oxides after thermal treatment (Chem. Commun. 2000, 2187-2188, DOI:10.1039/B005638N; J. Am. Chem. Soc. 2003, 125(12), 3593-3604; DOI:10.1021/ja0205737).
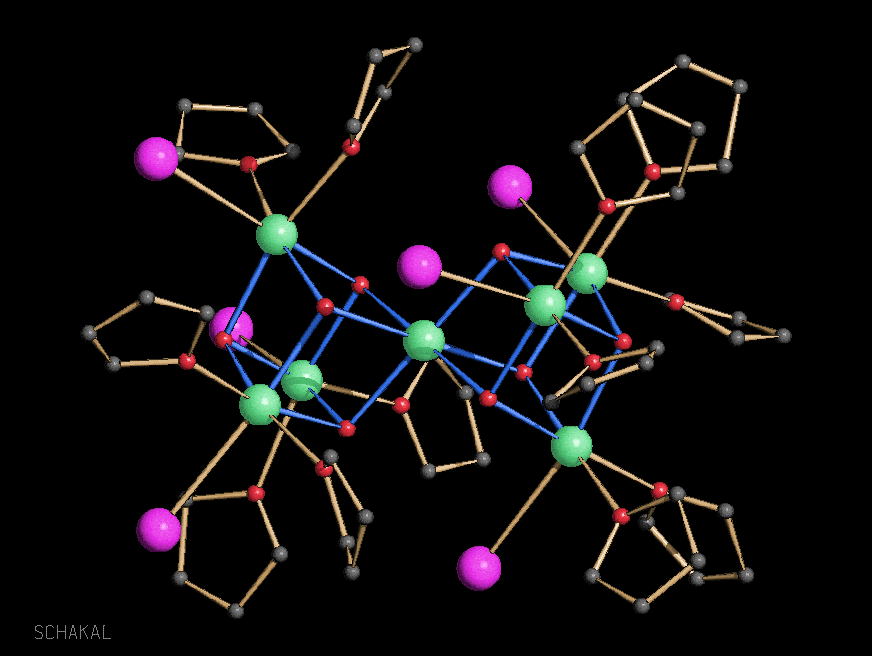
Ca7I6(OH)8(thf)12 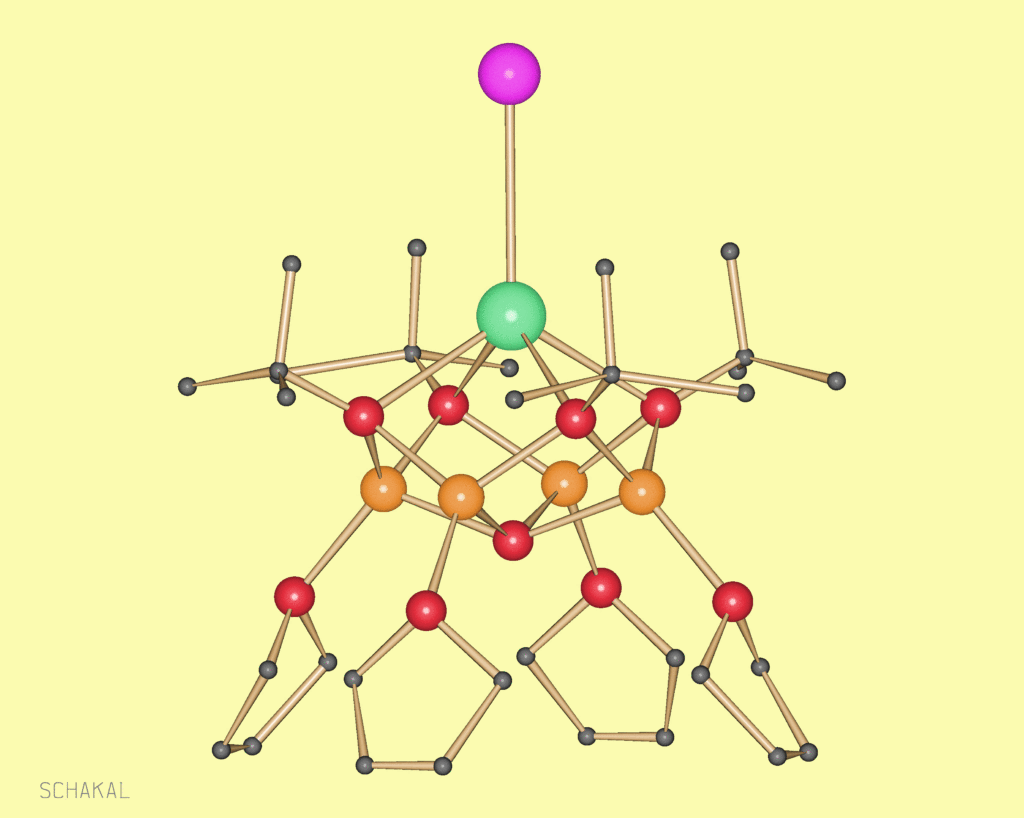
[IBa(OtBu)4{Li(thf)}4(OH)]
Molecular Alkaline Earth Iodides
The coordination geometry of alkaline earth metal iodide adducts is difficult to predict. We study such compounds in order to gain insight into the coordination behaviour of alkaline earth metal iodides in organic solvents with donor functions, such as THF and polyethers. Contrarily to the expected trans-iodides, we were also able to isolate cis-compounds with small I-M-I angles of ca. 90°, just as it is known for complexes of transition metal halides. ( Eur. J. Inorg. Chem. 2003, DOI: 10.1002/ejic.200300241; Z. Anorg. Allg. Chem. 2012, 638, 11, 1810-1819. DOI: 10.1002/zaac.201200231).

trans-[BaI2(thf)5] 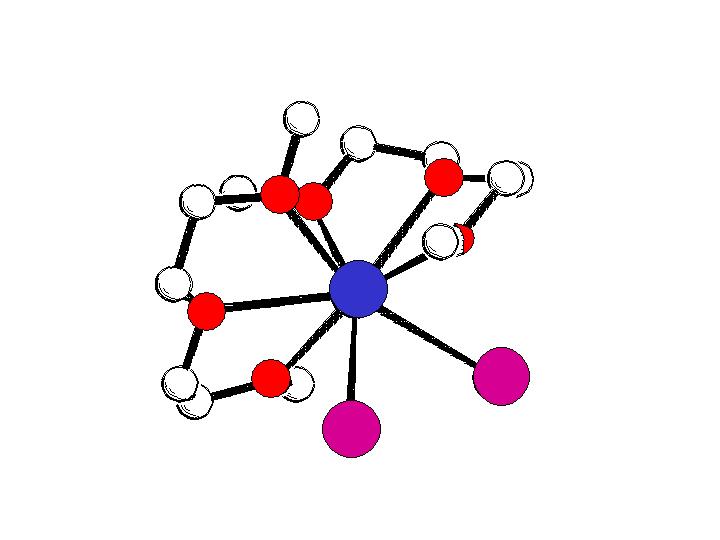
cis-[SrI2(diglyme)2]
Supramolecular H-bonded hybrid polymers
This part of our research deals with the problem of structure prediction. Using 2+ charged complexes of alkaline earth metal ions, featuring at least one water molecule in its coordination sphere with otherwise innocent O-donor ligands, we are able to predict the dimensionality of the formed supramolecular array. (Z. Anorg. Allg. Chem. 2000, 7, 1685-1691, ; Polyhedron, 2000, 19, 1783-1789, DOI:10.1016/S0277-5387(00)00466-6; Chem. Eur. J. 2001, 7, 2236-2244, ).
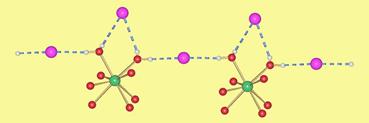
Towards ionic channels
Using calix[n]arenes and crown ethers as scaffolds, we firstly obtained molecular compounds, either polar structures at the molecular level, or alkali metal clusters. We also build up channel structures through which protons, alkali or alkaline earth metal cations can be transported. This is a rather recent project in our group and is interesting for charge transport mimics of biological systems with possible applications in miniaturization of “cables”. Recently, we were able to measure the kinetics of K+ and Na+ transport through crown ether based channel systems. (Polyhedron 2013, 52, 610-616, DOI:10.1016/j.poly.2012.08.004; Angew. Chem. Int. Ed. 2013, 17, 4682-4684, ).
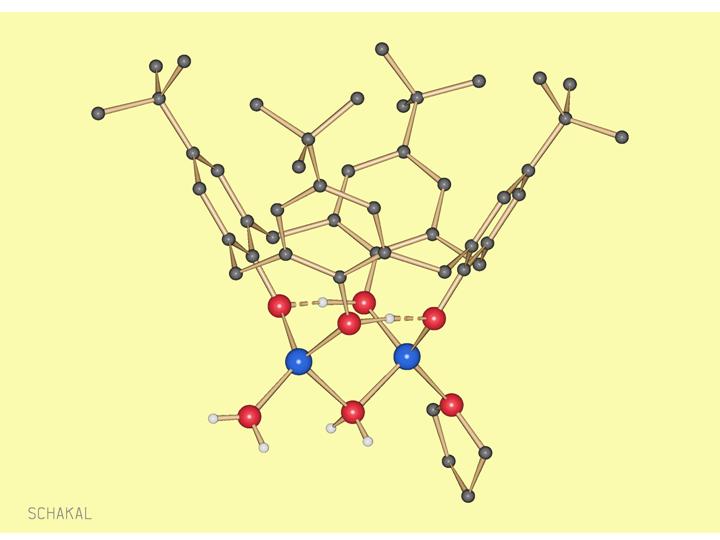
Li2(Calix[4]arene-2H)(H2O)2(THF) 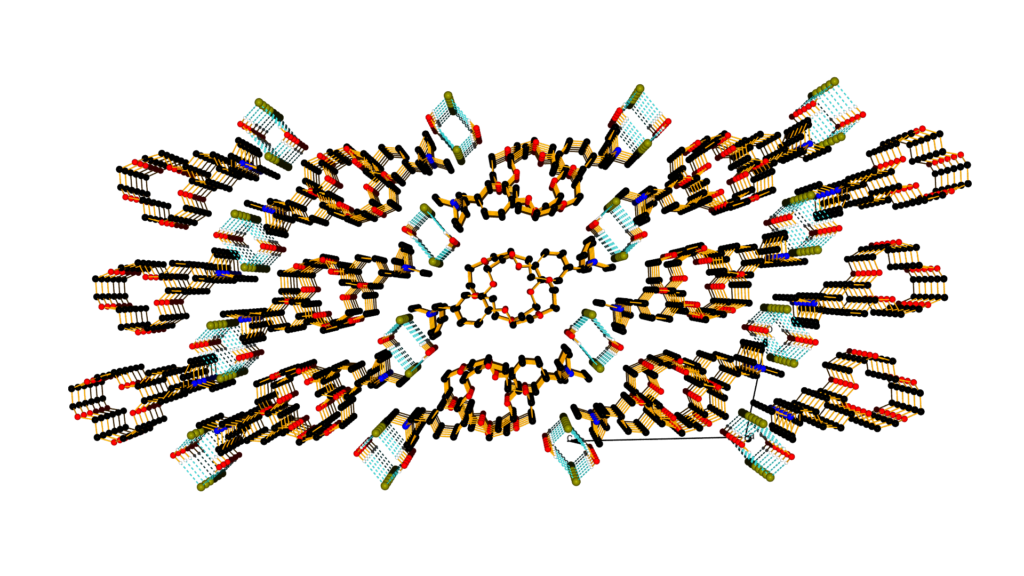
crown ether paking perspective 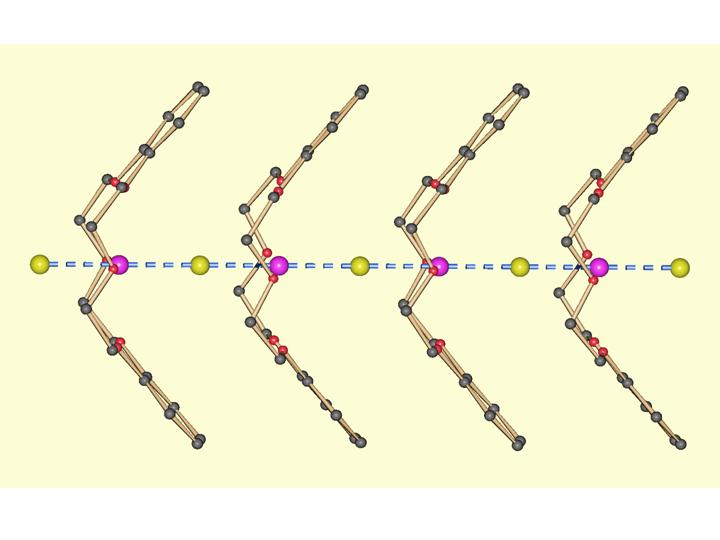
(H3O)(DB18C6)(H2O)I3
Water clusters, chains and proton transport
In our channel systems, but also in our coordination networks, we are interested in the synthesis, isolation and characterization of water molecules in form of clusters and chains. Thus, we are interested in water exchange reaction using D2O to replace H2O and to study the speed and mechanism of such transport. (Z. Anorg. Allg. Chem. 2002, 628, 171-178, ; Cryst. Growth & Design, 2005, 5(5), 1691-1694, DOI:10.1021/cg050201k).